Feeling stuck? Focus on patient safety, not compliance.
Sep 25, 2023
We are stuck in a system focused on compliance. Switch your focus to patient safety instead to get unstuck!
If you ask anyone in the medical device industry why they do risk management, you are likely to hear something like this:
Yes, it’s good business sense. Not incorporating risk management can lead to exorbitant costs (and the obvious, patient safety issues) for a company in corrections and removals, not to mention the lawsuits if and when a device fails.
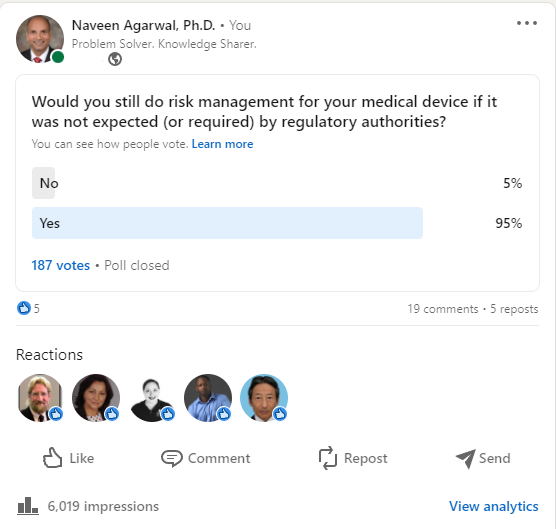
In a recent LinkedIn poll, a majority of respondents said they would still do risk management even when not required or expected by regulatory authorities.
Yet, it is a common feeling across the industry that risk management is hard. We have too many complex procedures, work instructions and forms. We spend too much time on documentation and not as much on deeply understanding the nature of risks associated with our medical device. As a result, we are frequently surprised by frequent recalls and warning letters.
We all have a strong desire to be patient-focused. Yet, we find ourselves stuck in a system that focuses mainly on compliance.
Yes, and we could already start to change if Notified Bodies and other agencies weren't "gatekeeping" risk management by demanding FMEA (not mandatory for medical devices), arguing about wordings, etc. I wonder if their expertise is based on too narrow training to allow critical thinking? Or is the narrow tolerance due to liability?
What if we made safety an explicit business goal? Would it inspire to do things differently?
Check out this article to learn more!
